-
Engineering and Architecture
Exams
Colleges
Predictors
Resources
-
Computer Application and IT
Quick Links
Colleges
-
Pharmacy
Colleges
Resources
-
Hospitality and Tourism
Colleges
Resources
Diploma Colleges
-
Competition
Other Exams
Resources
-
School
Exams
Ranking
Products & Resources
-
Study Abroad
Top Countries
Student Visas
-
Arts, Commerce & Sciences
Exams
Colleges
Upcoming Events
Resources
-
Management and Business Administration
Exams
Colleges & Courses
Predictors
-
Learn
Online Courses
Engineering Preparation
Medical Preparation
-
Online Courses and Certifications
Top Streams
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
Resources
-
Medicine and Allied Sciences
Colleges
Predictors
Resources
-
Law
Resources
Colleges
-
Animation and Design
Animation Courses
Colleges
Resources
-
Media, Mass Communication and Journalism
Exams
Colleges
Resources
-
Finance & Accounts
Top Courses & Careers
Colleges
Get Answers to all your Questions


All Questions
A sum of money under compound interest doubles itself in 4 years. In how many years will it become 16 times itself?
12 years
16 years
8 years
None of these
16 years
View Full Answer(6)
A certain loan amounts, under compound interest, compounded annually earns an interest of Rs.1980 in the second year and Rs.2178 in the third year. How much interest did it earn in the first year?
Rs.1600
Rs.1800
Rs.1900
None of these
xyz pqr abc year tt
View Full Answer(2)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

Spin only magnetic moment of an octahedral complex of  in the presence of a strong field ligand in BM is :
in the presence of a strong field ligand in BM is :
Option: 1 4.89
Option: 2 4.89
Option: 3 4.89
Option: 4 4.89
Option: 5 2.82
Option: 6 2.82
Option: 7 2.82
Option: 8 2.82
Option: 9 0
Option: 10 0
Option: 11 0
Option: 12 0
Option: 13 3.46
Option: 14 3.46
Option: 15 3.46
Option: 16 3.46
Electionic configuration of
In the preserce of a strong field ligand, pairing will take place
Thus there are no unpaired electrons and the spin only magnetic moment is zero.
Hence, the correct answer is option (3)
View Full Answer(1)The number of geometrical isomers found in the metal complexes
![\mathrm{\left[ PtCl _{2}\left( NH _{3}\right)_{2}\right],\left[ Ni ( CO )_{4}\right], \left[ Ru \left( H _{2} O \right)_{3} Cl _{3}\right] \text { and }\left[ CoCl _{2}\left( NH _{3}\right)_{4}\right]^{+}}](https://learn.careers360.com/latex-image/?%5Cmathrm%7B%5Cleft%5B%20PtCl%20_%7B2%7D%5Cleft%28%20NH%20_%7B3%7D%5Cright%29_%7B2%7D%5Cright%5D%2C%5Cleft%5B%20Ni%20%28%20CO%20%29_%7B4%7D%5Cright%5D%2C%20%5Cleft%5B%20Ru%20%5Cleft%28%20H%20_%7B2%7D%20O%20%5Cright%29_%7B3%7D%20Cl%20_%7B3%7D%5Cright%5D%20%5Ctext%20%7B%20and%20%7D%5Cleft%5B%20CoCl%20_%7B2%7D%5Cleft%28%20NH%20_%7B3%7D%5Cright%29_%7B4%7D%5Cright%5D%5E%7B+%7D%7D) respectively, are :
respectively, are :
Option: 1 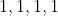
Option: 2 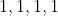
Option: 3 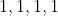
Option: 4 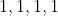
Option: 5 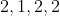
Option: 6 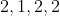
Option: 7 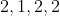
Option: 8 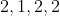
Option: 9 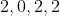
Option: 10 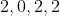
Option: 11 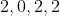
Option: 12 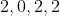
Option: 13 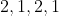
Option: 14 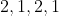
Option: 15 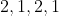
Option: 16 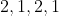
The complexes along with their geometrical isomers are given below:
(a) : Square planar
(b) : tetrahedral complex.
Does not show Geometrical isomerism.
(c) : Octahedral
(d) : Octahedral
Hence, the correct answer is option (3)
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE
The type of hybridisation and magnetic property of the complex ![\left[\mathrm{MnCl}_{6}\right]^{3-}](/latex-image/?%5Cleft%5B%5Cmathrm%7BMnCl%7D_%7B6%7D%5Cright%5D%5E%7B3-%7D) , respectively, are :
, respectively, are :
Option: 1 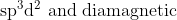
Option: 2 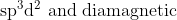
Option: 3 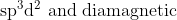
Option: 4 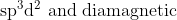
Option: 5 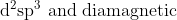
Option: 6 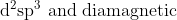
Option: 7 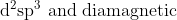
Option: 8 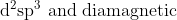
Option: 9 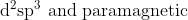
Option: 10 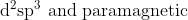
Option: 11 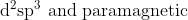
Option: 12 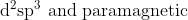
Option: 13 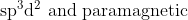
Option: 14 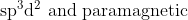
Option: 15 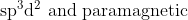
Option: 16 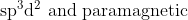
The complex contains
ion in a weak ligand field. The electronic arrangement is shown below :
Thus, the complex is hybridised with a paramagnetic nature.
Hence, the correct arswer is option (4)
View Full Answer(1)The overall stability constant of the complex ion is
. The overall dissociation constant is
. Then
is ___________(Nearest integer)
Given,
Stability constant
Hence, the answer is 5
View Full Answer(1)NEET 2024 Most scoring concepts
- Just Study 32% of the NEET syllabus and Score up to 100% marks
Arrange the following Cobalt complexes in the order of incresing Crystal Field Stabilization Energy (CFSE) value. Complexes :
Choose the correct option :
Option: 1
Option: 2
Option: 3
Option: 4
As we have learnt ,
The CFSE depends upon the nature of ligands if the metal cation is the same.
Also, cations having greater positive charge have a greater CFSE.
Thus, the given complexes can be arranged in order of their CFSE as
Hence, the correct answer is option (1)
View Full Answer(1)3 moles of metal complex with formula gives 3 moles of silver chloride on treatment with excess of silver nitrate. The secondary valency of CO in the complex is_______.
(Round off to the nearest integer)
The given complex is and its primary valency is 3 and secondary valency is 6 .
Hence, the answer is 6.
View Full Answer(1)Crack CUET with india's "Best Teachers"
- HD Video Lectures
- Unlimited Mock Tests
- Faculty Support

This is an abiotic resource a option grass B option forest C option mineral MCQ?
The correct option is C) Mineral.
Mineral is an Abiotic resource as it is not derived from living organisms.
Ankita and pooja are good friends but Poojas parent do not like girls going out without elders.discuss
Ankita and Pooja are good friends and wants to hang out together sometimes. However, Pooja's parents do not like girls going out without elders or adult supervision. According to me, they are right as the children are very young school going children. They should meet each other only at their homes and nearby places such as parks, eating joints only in presence of their parents. They should listen to the advice of their elders and follow their guidelines.
View Full Answer(1)JEE Main high-scoring chapters and topics
Study 40% syllabus and score up to 100% marks in JEE







